EMF of the cell: EMF of cell can be defined as the actual potential difference of the cell. The voltage that we are getting from the battery is reduced by the internal resistance of the battery.
You can see in the picture is the internal physical diagram of the cell. It contains a resistor and the cells voltage. Resistor that is connected here is not seen as this is not connected in the cell or it is outside the cell. It is the resistor that is of the cell. And emf of the cell is constant means that the emf of the particular cell does not change.
Ohms law: At constant temperature, voltage is directly proposal to the product of the current flowing and the net resistance.
V=IR
The electrochemical series
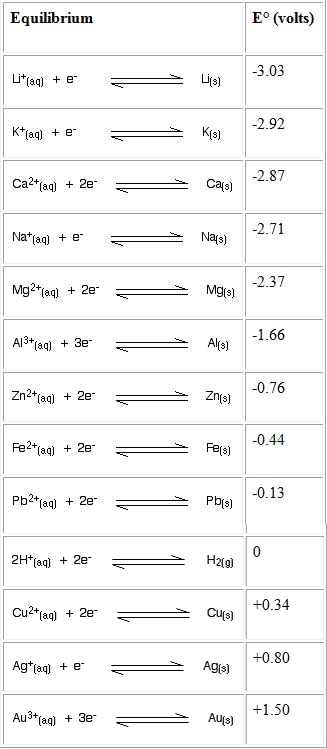
Finding the emf the cell is like this:
EMF = Positive charge giving compound is subtracted by the negative charge giving compound.
Positive charge giving compound I use is carbon. Which has E= 0.13
negetive charge giving compound I use is zinc. Which has E= -0.76
EMF = 0.13-(-0.76)
=0.13+0.76
=0.89V
Requirements for experiment: 1
Any metals like (aluminum foil), connecting wire, multimeter, alligator clip, cotton.
Procedure for experiment: 1
1.Take any alluminium foil present at your home.
 2. Take anything that absorbs water in it and place in top of it. Not very much little bit only.
2. Take anything that absorbs water in it and place in top of it. Not very much little bit only.
 3. Add water in it so that paper becomes wet.
3. Add water in it so that paper becomes wet.
 4.Connect multimeter into it positive wire or red wire upon the paper and negative wire or black wire on the alluminium foil.
4.Connect multimeter into it positive wire or red wire upon the paper and negative wire or black wire on the alluminium foil.

Note: Never ever try to meet the aluminium foil to the positive or red wire of multimeter nor negative or black wire in water.
 5. This is the small battery you can see in front of you can also make it. In your home if you want more current replace paper with charcoal. And add lemon in the water or vinegar. If you connect battery in series then you will get more voltage.
5. This is the small battery you can see in front of you can also make it. In your home if you want more current replace paper with charcoal. And add lemon in the water or vinegar. If you connect battery in series then you will get more voltage.
Advantage for experiment: 1
1.Lots of aluminium foil is wasted in the garbage after using it in the lunch box. This will give you aluminium free. You need not purchase it from shop asks your friend to give his used aluminium foil
2.It is eco-friendly.
3.Can be made anywhere. As it does not require any precious metal. Open your lunch box; take out your aluminium and then get start to make it.
4.Very easy to make.
5.Can be made anywhere.
Disadvantages for experiment: 1
1.As you should know that aluminium has a property of making a coat on its surface so that more reaction cannot be done. As you have made this battery, after sometime the reaction will stop and from there you will not be able to produce the charge. So you have to replace the aluminium very frequently.
Experiment 2
Requirements for experiment: 2
A battery, water, connecting wire, multimeter, cotton
Procedure for experiment: 2
1. Take battery disassemble it.
2. Only cut the back portion of the battery
3. Attach the 2 wire in the battery one in the positive side and other in the negative side.
4. Tape it so that the wire is fixed properly.
5. Insert cotton inside the battery so that the carbon rod does not move. If it moves it has a chance of toughing the zinc container.
 The picture you are able to see is the internal view of the battery
The picture you are able to see is the internal view of the battery
6. Add water to it. Make sure that you are not filling the container you are only wetting the cotton so that it becomes conductor.
 7.Connect the multimeter so that you can be able to get the voltage.
7.Connect the multimeter so that you can be able to get the voltage.
 8.You are getting 0.57v free but in the case of aluminium you were getting 0.2v because aluminum is makes a protective coat upon it so that is cannot be more decompose.
8.You are getting 0.57v free but in the case of aluminium you were getting 0.2v because aluminum is makes a protective coat upon it so that is cannot be more decompose.
 9. If you want to increase the potential difference than you have to reduce the internal resistance.
9. If you want to increase the potential difference than you have to reduce the internal resistance.
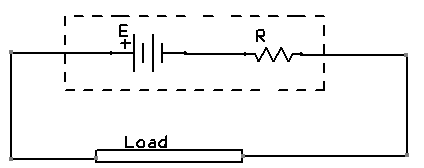 The R you can see in this picture is internal resistance. For decrease the internal resistance you have to add ionic compound so that the ions will decrease the resistance.
The R you can see in this picture is internal resistance. For decrease the internal resistance you have to add ionic compound so that the ions will decrease the resistance.
10.I add vinegar in the zinc container which makes my cell’s internal resistance less and I got 0.76V.

Advantages for experiment: 2
1.For making a clock to work you only have to make 2 batteries like this and connect them in series. (You can buy only zinc container from the shop)
2.Eco friendly. No pollution, no harmful metals all safe products.
Disadvantages for experiment: 2
1. Little costly.
2. Zinc decomposes and cannot be reused.
Calculation for experiment: 2
Finding the emf the cell is like this:
EMF = Positive charge giving compound is subtracted by the negative charge giving compound.
Positive charge giving compound I use is carbon. Which has E= 0.13
Negative charge giving compound I use is zinc. Which has E= -0.76
EMF = 0.13-(-0.76)
=0.13+0.76
=0.89V
The emf of the cell is 0.89V but it is showing 0.76V. It means that internal resistance till now didn’t become 1. For making it 1 you have to add such compound that will dissociate faster and will make internal resistance nearer to 1.
That compound is very high concentrated acid which easily lose H+ ion. But it is not safe so don’t ever think to do it without any expert. I have done it was 0.86V coming I will not give you this detail because it is very harmful. In a few seconds it can burn your skin or take your eye sight. So don’t think about it.
How all this has been Done.
You have read this that as the resistance will increase the potential difference will be decreased so you have to keep in mind to add that mixture which is stable and has less resistivity. I added bleaching powder in this so as to decrease its resistivity. Applications
Applications
Main application is that it is use to generate electricity with gold out of waste.If you are in that area where you are not getting the battery than open your lunch box. Take out the aluminum foil. Put it in a container add water into it. Aluminum will give you the negative charge and water will give you the positive charge.
Even though you can operate a calculator with this as shown in the figure.

Anyone who wants to not use any battery only he will use the free source he has to only collect aluminum from the waste then do the reaction by putting it in the beaker which contain water than add the terminal one in the aluminium as it produce negative charges and second terminal will be the water or you can add carbon rod there. Don’t or never add the both terminals same in the water first is aluminium means don’t dip aluminium attached with second terminal too as this will make aluminium as a electrode and there is no potential difference between same metal.
Electrochemistry
The anode oxidation half-reaction is Al + 3OH– Al(OH)3 + 3e– ?2.31 V.
The cathode reduction half-reaction is O2 + 2H2O + 4e– ? 4OH– +0.40 V.
The total reaction is 4Al + 3O2 + 6H2O ? 4Al(OH)3 + 2.71 V.
About 1.2 volts potential difference is created by these reactions, and is achievable in practice when potassium hydroxide is used as the electrolyte. Saltwater electrolyte achieves approximately 0.7 volts per cell.
Advantages:
1. It requires no new element. Can get all the things from the home.
2. It is harmless as water in metal vassals is harmless.
3. No pollution.
4. No hazards to nature.
5. Very easy and safe. Can be done anywhere.
6. Cost is very less….
Disadvantages:
1. Cannot give continuous energy as the metal have to be replaced.
2. Very slow process.
3. Less energy
4. Cannot operate high power circuits.
Project Source Code
Filed Under: Electronic Projects


Questions related to this article?
👉Ask and discuss on EDAboard.com and Electro-Tech-Online.com forums.
Tell Us What You Think!!
You must be logged in to post a comment.