As with any productive industry, the electronics sector is continually evolving and advancing. Currently, one of the biggest trends is miniaturization as the demand for smaller and lighter components grows, particularly in the PCB market.
 Semiconductor technology has also scaled down in size, leading to the miniaturization and integration of circuits. Now, except for inductors and a few passive components, it’s possible to manufacture most electronic devices on a single silicon chip.
Semiconductor technology has also scaled down in size, leading to the miniaturization and integration of circuits. Now, except for inductors and a few passive components, it’s possible to manufacture most electronic devices on a single silicon chip.
Silicon is the most common semiconductor material used for integrated circuit (IC) manufacturing although it is not the only one. Let’s investigate the different semiconductor materials used in IC manufacturing and investigate why silicon still dominates the semiconductor industry.
Materials
Solid-state devices are built using a type of semiconductor material, whereby electricity can flow through. Semiconductors have an electrical conductivity (10-7 to 10-13 mho/m) that’s between a good conductor (> 10-7 mho/m) and an insulator (< 10-13 mho/m).
Semiconductor materials are either a single crystal or a compound.
Examples of a crystal semiconductor:
- Silicon (Si)
- Germanium (Ge)
Examples of compound semiconductors:
- Gallium arsenide (GaAs)
- Gallium nitride (GaN)
- Gallium arsenide phosphide (GaAsP)
- Cadmium sulfide (CdS)
- Lead sulfide (PbS)
- Silicon carbide (SiC)
Originally, solid-state devices were manufactured using germanium but its strong temperature sensitivity was a drawback. As the manufacturing techniques improved, the use of silicon gradually became popular because of its thermal stability and availability.
As the electronic focus advanced from switching and control to computing and communication, the demand for high-speed devices increased. As a result, gallium arsenide was considered ideal as it offers a transistor rate that’s five times faster than one with silicon.
Silicon remains the most widely used semiconductor material, however, germanium arsenide is used exclusively for high-speed, very large-scale integration (VLSI) designs. Germanium is also still used for certain applications.
This trio — silicon, germanium, and gallium arsenide — are the most commonly used semiconductor materials. Others may still be used but only for specific assemblies or purposes.
IC manufacturing
There are reasons semiconductor materials are used in manufacturing electronic ICs. For one, such materials (including silicon, germanium, and gallium) offer a crystalline structure due to the covalent bonding between their atoms.
These covalent bonds are formed via shared valence electrons (which are the electrons in the outermost shell) with the adjacent atoms. Due to an abundance of ambient heat and light, many of these electrons gain enough kinetic energy to move freely throughout the material. In fact, it’s often said that the electrons move from the valence energy band to the conduction energy band.
 Since the electrons within the material are technically “free,” they can be pointed in a certain direction with the application of an external electric field or voltage.
Since the electrons within the material are technically “free,” they can be pointed in a certain direction with the application of an external electric field or voltage.
To ensure the ideal electrical and physical properties, semiconductor materials are typically refined to their purest form — and, as such, they’re labeled, intrinsic materials. Technology has made it possible to obtain only one impurity atom in 10 billion within a refined, intrinsic crystal.
What’s interesting about these intrinsic semiconductor materials is that their conductivity and electrical properties can be easily changed and controlled by adding impurity atoms to their lattice structure. For example, the addition of just one impurity atom per 10 million atoms can change its conductivity fairly dramatically.
This process is referred to as doping and, afterward, a semiconductor material is called extrinsic material. There are two types of extrinsic materials: n-type and p-type. Silicon and germanium are both tetravalent, meaning they have a valence of four. For gallium arsenide, gallium is trivalent and arsenide is pentavalent (a valence of five).
Regardless of their number of valence electrons, it’s worth noting that silicon, germanium, and gallium arsenide all have a crystalline structure due to covalent bonding.
Now, when a pentavalent impurity such as arsenic, phosphorous, or antimony is added to an intrinsic semiconductor, it becomes an n-type extrinsic material. The pentavalent atoms give an extra electron to the crystal, which acts as a free-charge carrier.
Diffused impurities with five valence electrons are called donor atoms. The diffusion of impurity atoms does not change the overall electrical charge of the material as the number of electrons remains the same as the positive charge in the nuclei. Rather, the impurity atoms produce another discrete energy band between the valence and the conduction band. This is called donor level.
If only one impurity atom in 10 million atoms of intrinsic material is added — which initially only had one impurity atom in 10 billion atoms — the concentration of free-charge carriers is changed by a factor of 1,000.
Similarly, the p-type material is produced by adding trivalent impurities, such as gallium, boron, or indium to an intrinsic material. The trivalent impurities have one insufficient electron to fully complete covalent bonding in the new crystal structure. This vacancy acts as a positive charge and is called, hole. The free electrons in the p-type extrinsic material will continually fill up one or the other hole, always leaving behind a hole.
When an external electric field is applied to an n-type or p-type material, electrons and holes present as free-charge carriers are driven by the direction of the field and resulting in the conduction of the electric current.
This electric current is the result of the movement of the electrons and holes. In the n-type materials, the electrons are majority-charged carriers while the holes are the minority-charged carriers. In the p-type material, it’s reversed. The holes are the majority-charged carrier while electrons are the minority-charged carriers.
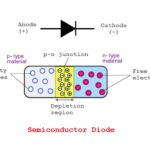
Solid-state electronic devices are constructed by p-type and n-type materials. Much like in a diode, one n-type and one p-type of the material are sandwiched to form a junction with the conduction of current between the two terminals. In a transistor, two p-types and one n-type, or one p-type and two n-types are sandwiched to form two junctions between them — and the conduction of current flows across three terminals.
This is similar to how all other solid-state devices are manufactured.
Material comparisons
As mentioned, silicon, germanium, and gallium arsenide are currently the most widely used intrinsic semiconductors for making ICs.
The free electrons in the intrinsic material are called intrinsic carriers and are as follows for these materials (per-cubic-centimeter):
- Silicon: 1.5*1010
- Germanium:2.5*1013
- Gallium arsenide:1.7*106
Another important factor is the relative mobility of the intrinsic carriers as the ability of free carriers to move through the material is determined by it. By definition, relative mobility is the average charge carrier velocity when subjected to an electric field. It’s measured in meters per second divided by volts per meter.
The relative mobility for these materials is as follows:
- Silicon: 1500 cm2/Vs
- Germanium: 3900 cm2/Vs
- Gallium arsenide: 8500 cm2/Vs
You’ll note that gallium arsenide has the least number of intrinsic carriers but the highest relative mobility, which is why devices made with GaAs offer the highest response speed.
The electrical properties of the semiconductor materials depend on the number of free carriers and their relative mobility. Additionally, the thermal and optical behavior of the semiconductor materials largely depends on the gap between their valence and conduction bands.
Semiconductors have a negative temperature coefficient of resistance while conductors have a positive temperature coefficient of resistance. The smaller the band gap, the lower a material’s thermal stability.
The band gap is measured in electron volts as follows:
- Silicon: 1.14 eV
- Germanium: 0.67 eV
- Gallium arsenide: 1.43 eV
Germanium is not very thermally stable because of its smaller band gap. This is why it’s typically selected for use in heat and light-sensitive devices.
The greater the band gap, the more thermally stable the material — meaning it’s also more likely to emit energy in the form of light instead of heat. For this reason, GaAs is often used in the design of light-emitting diodes.
Thermally stable materials are also more suitable for computing and communication applications.
Silicon choices
Silicon wafers are still the most commonly used material for IC manufacturing. There are three major reasons for this.
1. Silicon is abundant and easily obtained. What’s more, is the refining process for silicon has drastically improved over the last couple of decades so it’s possible to obtain intrinsic silicon with extremely high purity levels compared to other semiconductor materials.
2. Modern electronic applications are based on computing and communication rather than switching and control. These applications require circuitry to be thermally stable, which means silicon (with a band gap of 1.14 eV) is an ideal match, especially compared to other compound semiconductors.
3. Silicon has a history. The first silicon transistor was designed in 1954 so chip designers are familiar with it and have devised highly efficient chip designs and silicon networks over the years. This is also why it’s more cost-effective to design an integrated circuit on a silicon substrate compared to any other semiconductor material.
Eventually, gallium arsenide might fully replace silicon and currently serves as an alternative for VLSI and ULSI designs. GaAs offer five times greater speeds than silicon circuits, so as the demand for high-speed circuits intensifies, it may become more attractive.
This is particularly true as technology and network designs evolve — which, if the past is an indication of the future, it’s only a matter of time.
You may also like:
Filed Under: Tech Articles



Questions related to this article?
👉Ask and discuss on Electro-Tech-Online.com and EDAboard.com forums.
Tell Us What You Think!!
You must be logged in to post a comment.