In current technology scenario, monitoring of gases produced is very important. From home appliances such as air conditioners to electric chimneys and safety systems at industries monitoring of gases is very crucial. Gas sensors are very important part of such systems. Small like a nose, gas sensors spontaneously react to the gas present, thus keeping the system updated about any alterations that occur in the concentration of molecules at gaseous state.
Gas sensors are available in wide specifications depending on the sensitivity levels, type of gas to be sensed, physical dimensions and numerous other factors. This Insight covers a methane gas sensor that can sense gases such as ammonia which might get produced from methane. When a gas interacts with this sensor, it is first ionized into its constituents and is then adsorbed by the sensing element. This adsorption creates a potential difference on the element which is conveyed to the processor unit through output pins in form of current. What is this sensing element? Is it kept in some chamber or is kept exposed? How does it get current and how it is taken out? Let’s find out in this Insight!!!

Fig. 1: Image Showing A Typical Gas Sensor
The gas sensor module consists of a steel exoskeleton under which a sensing element is housed. This sensing element is subjected to current through connecting leads. This current is known as heating current through it, the gases coming close to the sensing element get ionized and are absorbed by the sensing element. This changes the resistance of the sensing element which alters the value of the current going out of it.
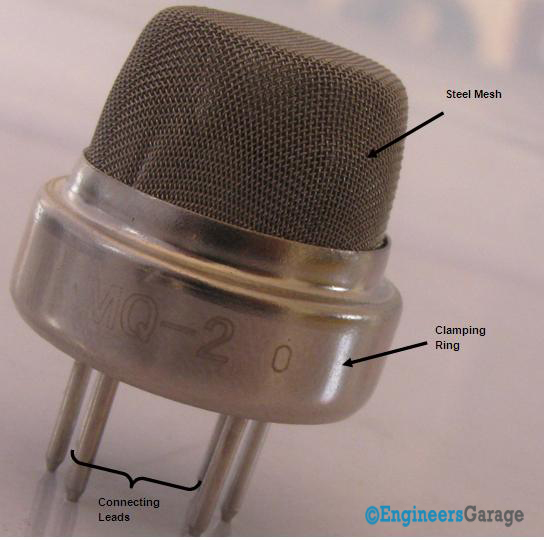 Fig. 2: Image Showing Various Parts of A Gas Sensor
Fig. 2: Image Showing Various Parts of A Gas Sensor
Image 01 shows externals of a standard gas sensor module: a steel mesh, copper clamping ring and connecting leads. The top part is a stainless steel mesh which takes care of the following:
1. Filtering out the suspended particles so that only gaseous elements are able to pass to insides of the sensor.
2. Protecting the insides of the sensor.
3. Exhibits an anti-explosion network that keeps the sensor module intact at high temperatures and gas pressures.
In order to manage above-listed functions efficiently, the steel mesh is made into two layers. The mesh is bound to rest of the body via a copper plated clamping ring.

Fig. 3: Steel Mash Used In Gas Sensor
The connecting leads of the sensor are thick so that sensor can be connected firmly to the circuit and sufficient amount of heat gets conducted to the inside part. They are casted from copper and have tin plating over them. Four of the six leads (A, B, C, D) are for signal fetching while two (1,2) are used to provide sufficient heat to the sensing element.
The pins are placed on a Bakelite base which is a good insulator and provides firm gripping to the connecting leads of the sensor.
Internal Features
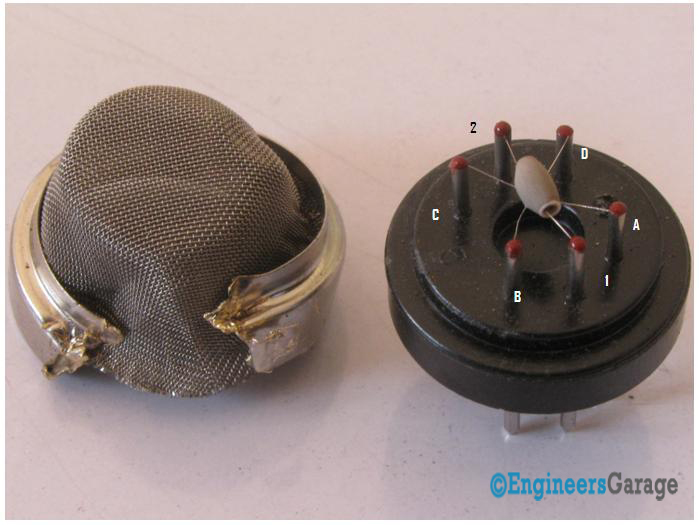
Fig. 4: Inside View of Gas Sensor After Removal of Steel Mash
The top of the gas sensor is removed off to see the internals parts of the sensor: sensing element and connection wiring. The hexapod structure is constituted by the sensing element and six connecting legs that extend beyond the Bakelite base.

Fig. 5: Image Showing Hexapod Structure Inside A Gas Sensor
Image4 shows the hollow sensing element which is made up from Aluminum Oxide based ceramic and has a coating of tin oxide. Using a ceramic substrate increases the heating efficiency and tin oxide, being sensitive towards adsorbing desired gas’ components (in this case methane and its products) suffices as sensing coating.
The leads responsible for heating the sensing element are connected through Nickel-Chromium, well known conductive alloy. Leads responsible for output signals are connected using platinum wires which convey small changes in the current that passes through the sensing element. The platinum wires are connected to the body of the sensing element while Nickel-Chromium wires pass through its hollow structure.
Ceramic Sensing Element

Fig. 6: Ceramic Sensing Element Present Inside A Gas Sensor
While other wires are attached to the outer body of the element, Nickel-Chromium wires are placed inside the element in a spring shaped. Image 5 shows coiled part of the wire which is placed on the inside of the hollow ceramic.
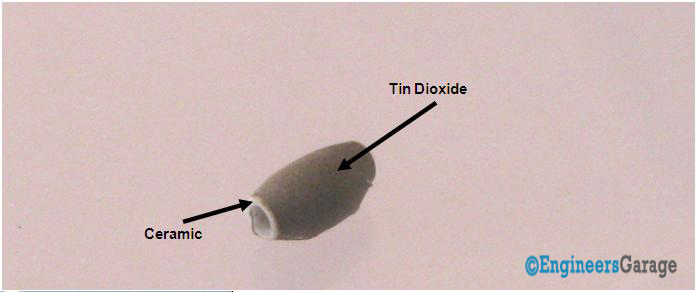
Fig. 7: Closer Look At The Ceramic Element
Image06 shows the ceramic with tin dioxide on the top coating that has good adsorbing property. Any gas to be monitored has specific temperature at which it ionizes. The task of the sensor is to work at the desired temperature so that gas molecules get ionized. Through Nickel-chromium wire, the ceramic region of the sensing element is subjected to heating current. The heat is radiated by the element in the nearby region where gases interact with it and get ionized. Once, ionized, they are absorbed by the tin dioxide. Adsorbed molecules change the resistance of the tin dioxide layer. This changes the current flowing through the sensing element and is conveyed through the output leads to the unit that controls the working of the gas sensor.
Filed Under: Insight


Questions related to this article?
👉Ask and discuss on Electro-Tech-Online.com and EDAboard.com forums.
Tell Us What You Think!!
You must be logged in to post a comment.