The battery stores electrical energy in form of chemical energy and the chemical energy again able to convert into electrical energy. The conversion of chemical energy to electrical energy is called discharging. The chemical reaction during discharge makes electrons flow through the external load connected at the terminals which causes the current flow in the reverse direction of the flow of the electron.
Some batteries are capable to get these electrons back to the same electron by applying reverse current, This process is called charging. The capable batteries to get back electrons in the same electrode are called chargeable and if they are not capable to do this, are called non-rechargeable.
In a battery, the electrode where reduction occurs is called the cathode and where oxidation occurs is called the anode.
There are three types of batteries in the market which are commonly used as rechargeable batteries.
- Lead-Acid batteries
- Ni-Cd batteries
- Ni-MH batteries
- Li-ion batteries
Lead-Acid batteries
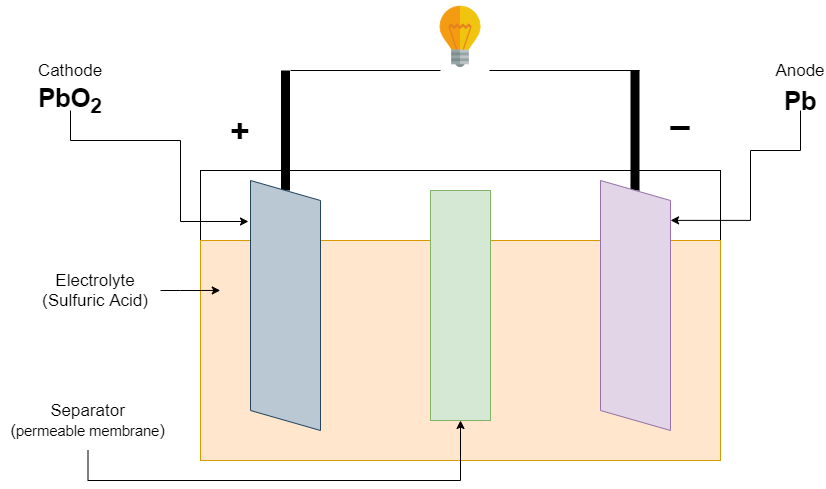
Firstly, the Lead-acid battery is invented in 1859 by French physicist Gaston Plante. it’s a negative electrode (anode) is made of spongy or porous lead. The Positive electrode (cathode) consists of lead oxide. Both anode and cathode electrodes are immersed in an electrolytic solution of sulfuric acid and water (dilute sulfuric acid).
The chemically permeable membrane separates the two electrodes which prevent short circuit. This membrane also prevents electrical shorting through the electrolyte.
The lead-acid battery has a nominal voltage of about 2v, it can vary from 1.8v at loaded at full discharge to 2.40v in an open circuit at full charge. The calculation of charging voltage can be done with voltage 2.40v/cell. 12v lead acid battery can be made from 6 cells connected in series. The current capacity is totally dependent upon manufacturer and size, it can vary from approximately 1Ah to almost 150Ah. For example, 12V with 4Ah or more can be used in vehicle ignition, 12v with 150Ah battery can be used for an inverter.
Discharging of Lead-Acid batteries
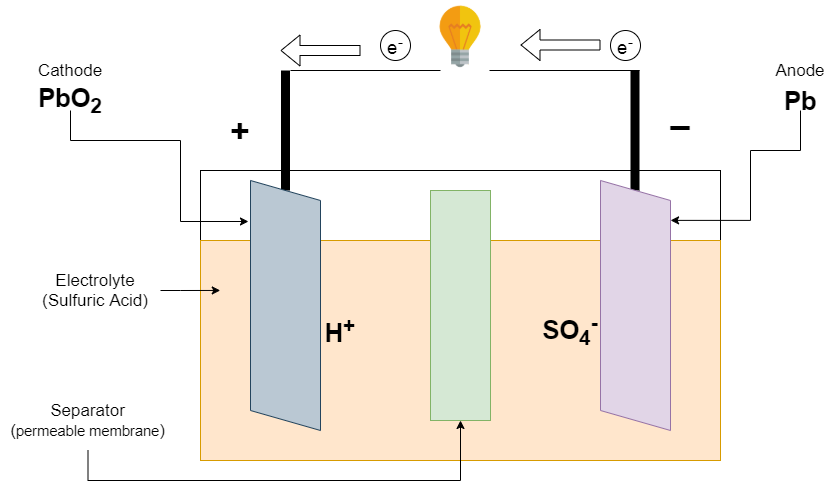
When the battery is connected to a load, The battery begins to discharge. The sulfuric acid (H2SO4) breaks into two parts hydrogen (2H++) ions and sulfate ions (SO4— ). The hydrogen ion takes an electron from the positive electron and sulfate ions give an electron to the negative plate. This inequality of electron cause flow of current at external load to balance the inequality of electrode.
Reaction at the Negative electrode
Pb + HSO4– ———-> PbSO4 + H+ + 2e–
Reaction at the Positive electrode
PbO2 + HSO4– + 3H+ + 2e– ———-> PbSO4 + 2H2O
Overall Reaction
Pb + PbO2 + 2H2SO4 ———-> 2PbSO4 + 2H2O
The Discharge of the lead-acid battery causes the formation of lead sulfate (PbSO4) crystals at both the positive electrode (cathode) and the negative electrode (anode), and release electrons due to the change in valence charge of the lead. This formation of lead sulfate uses sulfate from sulfuric acid which is an electrolyte in the battery. This makes sulfuric acid less concentrated at full discharge both electrodes being covered with lead sulfate and water, there is no sulfuric acid surrounding the electrode. The electrode is fully covered with the same material at full discharge, The material is lead sulfate so there is no chemical potential or voltage between the two electrodes. In practice, there is cutoff voltage to stop the discharge, long before this point.
Let’s find out the discharge rate, lead-acid battery usually specified at the 8, 10, or 20 hours rate which is C/8, C/10, C/20. if you find ratings on battery 12v 200Ah/10h or C/10.
Discharge Rate is C/10 = 200 Ah / 10 h = 20A
The C/10m it cut of voltage after a specific time, here 10 hours for C/10.
Charging of Lead-Acid batteries
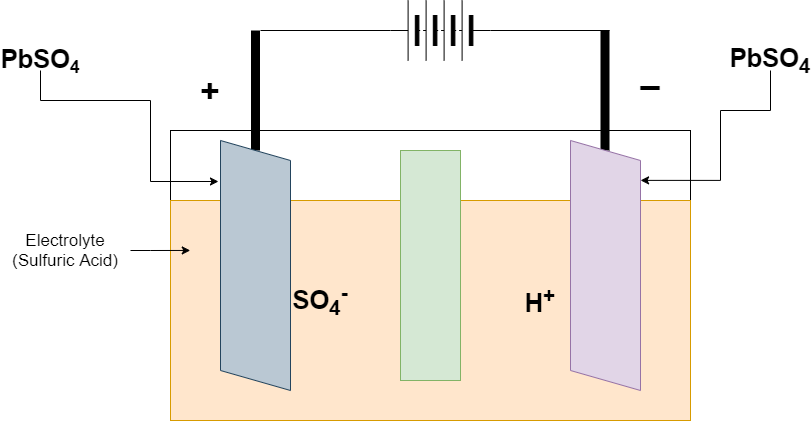
The Charging begins when the Charger is connected at the positive and negative terminal. the lead-acid battery converts the lead sulfate (PbSO4) at the negative electrode to lead (Pb) and At the positive terminal, the reaction converts the lead sulfate (PbSO4) to lead oxide. The chemical reactions revers from discharging process
Reaction at the Positive electrode
PbSO4 + 2H2O ———-> PbO2 + HSO4– + 3H+ + 2e–
Reaction at the Negative electrode
PbSO4 + H+ + 2e– ———-> Pb + HSO4–
Overall Reaction
2PbSO4 + 2H2O ———-> Pb + PbO2 + 2H2SO4
The charging current electrolyzes the water from the electrolyte and both hydrogen and oxygen gas are produced this process called “gassing” of the battery. This gassing raises several problems in the battery. This is unsafe due to the explosive nature of hydrogen produced. This also reduces water in the battery which can be manually replaced but it adds a maintenance factor. The gassing may cause shading of active material from electrolyte which permanently reduces the battery capacity that’s why a battery should not regularly charge above the voltage which causes gassing. Gassing voltage can be changed with the charge rate
There are several methods to charge the lead-acid batteries. But we should use the best method to reduce the chance of gassing, to obtain maximum battery service life and capacity. The list of charging methods Given below.
- Constant voltage:- As a name, this method will provide constant voltage till the current taking by the battery go to zero. It takes a long time
- Constant current:- As a name, this method will provide constant current till the voltage reach its defined gassing voltage. It also takes a long time.
- multi-step constant current:- In this method the charging current is constant at voltage reaches gassing voltage then-current start to reduced in steps to maintain the voltage below gassing voltage. This charger is complicated to build.
- Modified constant voltage-current:- In this method battery charged in three stages. The first stage is the constant current stage, In this current applied to the battery till voltage reached its defined gassing voltage. In the second stage, the voltage is constant till the current decreases the value of about 0.1C20 (also known as C20/10 ). The voltage is will reduce to floating voltage ( generally 2.25v to 2.27v) to maintain the battery charged.
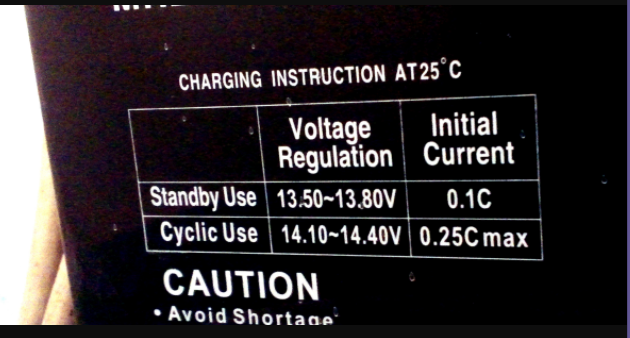
The charging current and gassing voltage can be found on the label on the battery as you can see in the image there are two modes to choose to charge voltage and current which are standby use and cyclic use. Cyclic use is the use of a battery where the need to charge and discharge quickly. Standby use is where the battery is charged already and when needed it used. 0.1C means multiply 0.1 by the total capacity of the battery. If you have a 40Ah battery means 0.1C is 0.1 x 40 = 4A. Same for 0.25C = 0.25 x 40 = 10A.
The life cycle of lead-acid batteries
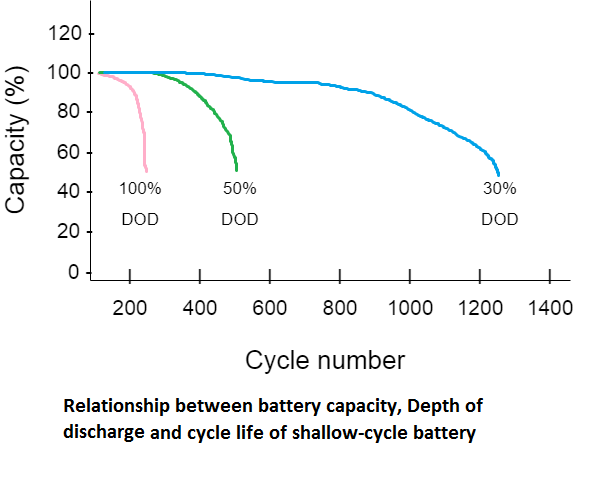
The lead-acid battery life cycle depends upon various factors. Generally, we say its charging/discharging cycle is about 200 to 300 cycles for shallow cycle batteries, but this number can increase or decrease. The life cycle of this battery depends upon three factors depth of discharge, correct charging cycle, and temperature. Deep cycle battery can maintain a life cycle of around 1000, but what are sallow cycle and deep cycle? , you can find it below.
- Depth of discharge- depth of discharge (DOD) means how much your battery is discharged. Let’s assume you have a 100Ah battery, you have discharged it for 20 minutes for 50A so the depth of discharge is given below.
Covert time in hour = 20/60
Calculate discharge time = 50 x 20/60 = 16.7 A
Depth of discharge = ( discharge / capacity ) x 100 = ( 16.7/100 ) x 100 = 16.7 %
There are two types according to DOD of battery, battery which has DOD capability of more than 50 % is called Deep cycle battery, and battery which cut off before 50 % of DOD is called shallow cycle battery. The deep cycle battery able to maintain a life cycle of around 1000 even if the Depth of discharge is more than 50 %, but the shallow cycle battery can very their life cycle as graph given below.
- Charging: If charging is not correct then it cause overcharging or undercharging which also reduces the capacity of your battery.
- Temperature: the life cycle also affected by temperature the capacity of the battery reduced at low-temperature operation, high-temperature operation increase the aging rate of the battery.
Ni-Cd batteries
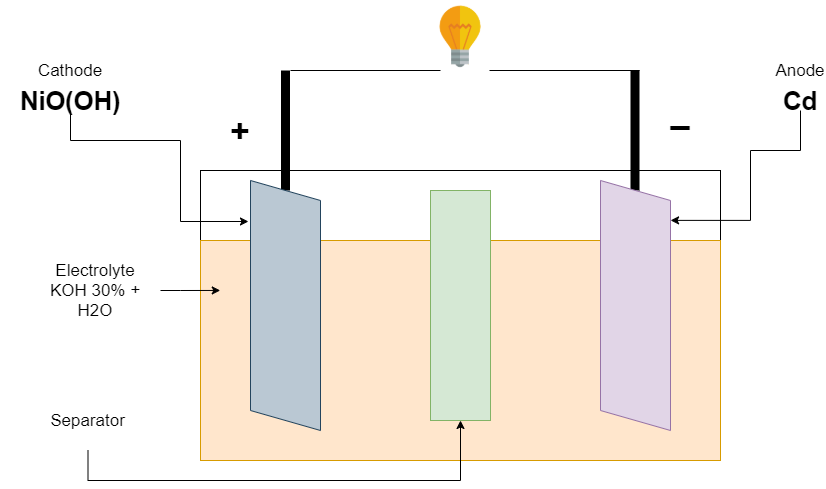
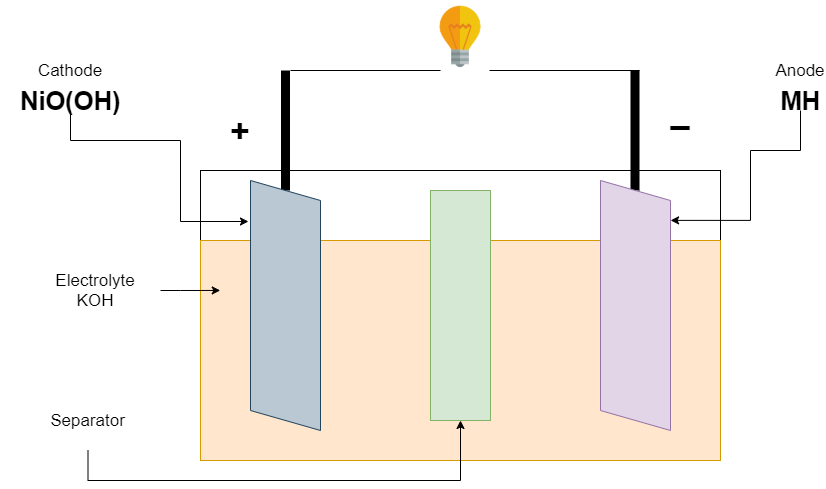
Firstly, the Ni-Cd battery is invented in 1899 by Waldermar Jungner. its positive electrode (Cathode) made of nickel oxide hydroxide (NiO(OH)), and a negative electrode (anode) made of Metallic cadmium (Cd). The electrolyte used is 30 percent potassium hydroxide (KOH) in distilled water. The electrolyte level maintained just above the top of the electrode. There are no appreciable changes that occur in the electrolyte during charging and discharging.
This battery has a discharge/charge cycle is about 2000 cycles. Its nominal voltage is 1.2v per cell, and its fully charged voltage is 1.55v. it is fully discharged when the voltage is down to 1.1v. Voltage can be increased by connecting cells in series. The manufacturer defines the capacity of the battery, commonly AA battery available is near 1000mAh.
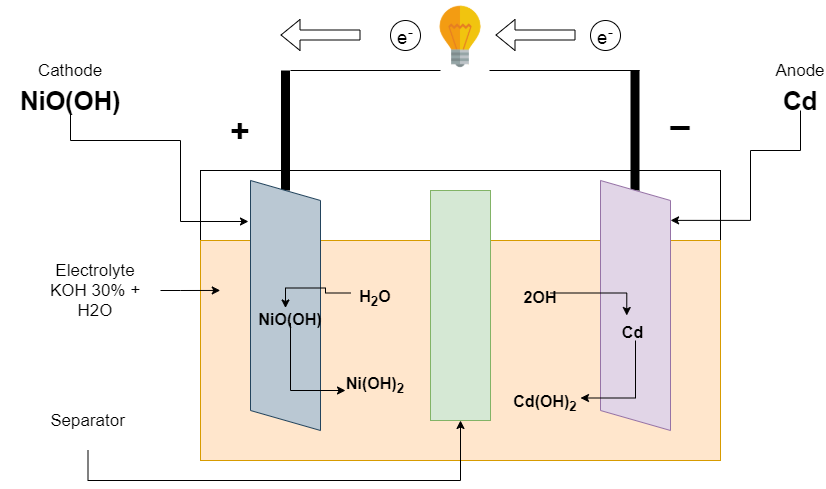
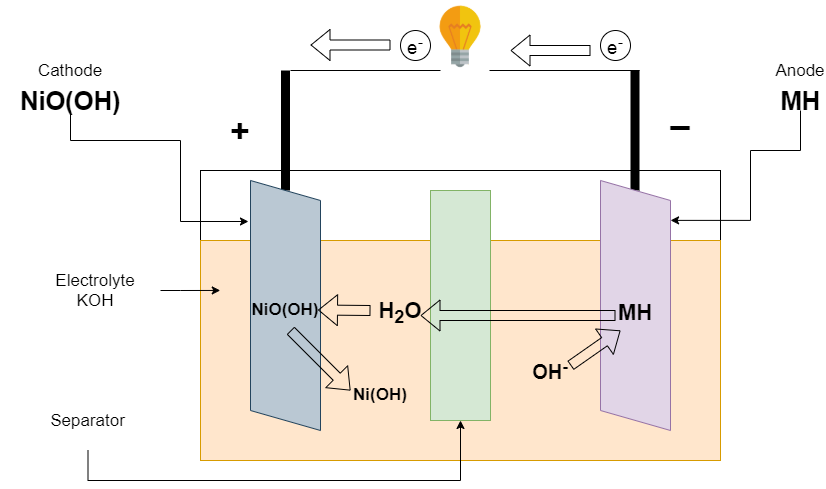
Discharging of Ni-Cd batteries
The battery begins to discharge when the load is connected at terminals. The (KOH) potassium hydroxide is dissociated into potassium (K+) and hydroxyl (OH–) ions. The hydroxyl (OH–) ions go towards the negative electrode. The negative electrode releases the electron and the Positive electrode receives it by external connection. This causes current to flow through the load from the positive to the negative electrode.
Reaction at the Negative electrode
Cd + 2OH ———-> Cd(OH)2 + 2e–
Reaction at the Positive electrode
NiO(OH) + H2O + 2e– ———-> Ni(OH)2 + OH–
Overall Reaction
Cd + 2NiO(OH) + 2H2O ———-> 2Ni(OH)2 + Cd(OH)2
The discharge rate is varied by the size of the battery common AA battery can deliver a current of approximately 1.8 amperes and a D-size battery able to deliver approximately 3.5-ampere current.
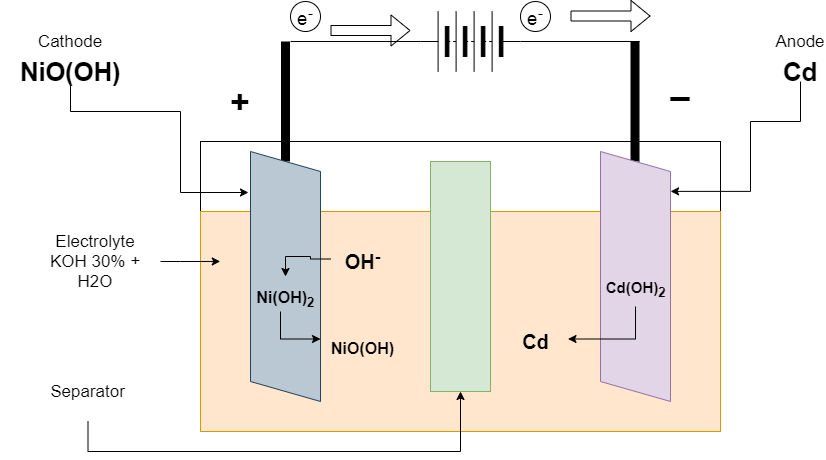
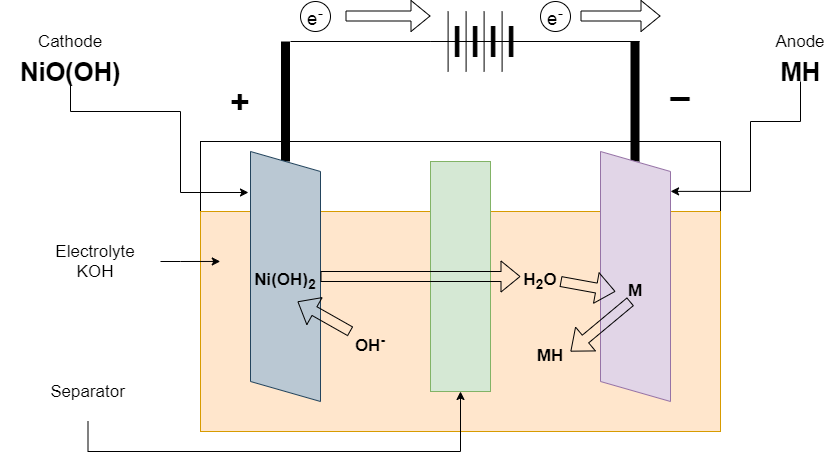
Charging of Ni-Cd batteries
At the time of charging, The charger is connected at terminals. The reaction is reversed from discharging. The positive electrode converts Ni(OH)2 to NiO(OH) and releases the electron. The electron is taken by a negative electron from external connections and it converts Cd(OH)2 to Cd.
Reaction at the Positive electrode
Ni(OH)2 + OH– ———-> NiO(OH) + H2O + 2e–
Reaction at the Negative electrode
Cd(OH)2 + 2e– ———-> Cd + 2OH
Overall Reaction
2Ni(OH)2 + Cd(OH)2 ———-> Cd + 2NiO(OH) + 2H2O
At the end of the charging cycle, the cells emit gas and this will also occur when the cell is overcharged. From this gas, the water in the electrolyte will decompose into hydrogen at the negative electrode and oxygen at the positive electrode. This gassing depends upon the voltage used to charge the cell and temperature. To Fully charge the Ni-cd battery slightly gassing must take place, thus some water is used from the concentration of electrolyte.
There are two methods to charge the ni-cd batteries. Slow charge and fast charge.
- Slow charge:- Slow charge current is about 0.1C it will not damage the cell when it fully charged. This method is also used to overcome the self-discharge of ni-cd batteries.
- Fast charging:- In fast charging the cell is charged at a constant current of about 1C. C is the capacity of the battery, if you are using a 4Ah battery then 1C means 1 x 4 = 4A. Once it fully charged which can be detected by the charge detection algorithm given below. The current will be reduced to 0.1C and a trickle charge is applied. Trickle charge is the charging at the same rate at which the battery will self-discharge. This will maintain the battery at a full charge state.
The full charge detection algorithm can use two factors Negative delta V or temperature.
If the algorithm using temperature to detect then the temperature will be 45 degrees for fast charging and 50 degrees for slow charging.
In the Negative delta V Detection algorithm, the voltage drops after a full charge. The detection of this drop can be used to detect the full charge state. This method called Negative delta V. This method provides precise full charge detection.
Ni-MH batteries
Firstly, the Ni-MH battery is invented in 1967 by Battelle-Geneva Research Center. Then it introduced in 2005 by Sanyo, branded Eneloop. In this battery positive electrode (cathode) made of nickel oxide hydroxide and a negative electrode made of hydrogen-absorbing alloy (Metal hydride). The electrolyte used is potassium hydroxide (KOH) concentrated with distilled water.
This battery has a discharge/charge cycle is about 180 – 2000 cycles. This depends upon various factors, how you are charging or discharging the battery.
This battery is almost similar to the Ni-Cd battery. The nominal voltage for the Ni-MH battery is 1.2V for a single cell. But at full charging, the voltage is 1.5V, and the full discharge voltage is 1.0V. The current capacity of this battery varies on its size, a AA battery can available near 2000mAh.
Discharging of Ni-MH batteries
The reaction of discharging begins when the load is connected at terminals. The Metal hydride (MH) reacts with OH ions to form M and water, and also releases an electron. The electron is taken by NiO(OH) through an external load. This causes electricity to flow through the load.
Reaction at the Negative electrode
MH + OH– ———-> M + H2O + e–
Reaction at the Positive electrode
NiO(OH) + H2O + e– ———-> Ni(OH)2 + OH–
Overall Reaction
NiO(OH) + MH ———-> Ni(OH)2 + M
Normally Ni-MH battery discharges at the rate of 3C (where C is the capacity of battery but the high-quality battery can discharge up to a rate of 15C.
Charging of Ni-MH batteries
At the time of charging, the charger is connected at the terminal of the battery the reactions of charging are reverse from discharging reactions. The positive electrode converts Ni(OH)2 to form NiOOH, water and releases an electron. This electron is taken by the negative electrode from the external wire and it from MH again.
Reaction at the Positive electrode
Ni(OH)2 + OH– ———-> NiO(OH) + H2O + e–
Reaction at the Negative electrode
M + H2O + e– ———-> MH + OH–
Overall Reaction
Ni(OH)2 + M ———-> NiO(OH) + MH
The Ni-MH battery charging chemistries utilize constant current and constant voltage algorithms that can be broken into four parts given below.
- Trickle Charge:- When the battery is deeply discharged it is below 0.9 V per cell. the constant current of 0.1C maximum used to charge the battery is called trickle charge.
- Constant Current:- When voltage is above 0.9V per cell the constant current is applied in the range of 0.2 C to 1C to perform constant current charging.
- Charge Termination:- Full charge of the battery can be detected by a full charging detection algorithm which is explained below. After full charge trickle charging is used at the rate of self-discharge to maintain full charge of the battery.
The full charge detection algorithm can use two factors Negative delta V or temperature.
If the algorithm using temperature to detect then the temperature will be 45 degrees to 50 degrees to detect full charge.
In the Negative delta V Detection algorithm, the voltage drops after a full charge. The detection of this drop can be used to detect the full charge state. This method is called Negative delta V. This method provides precise full charge detection.
Li-ion batteries
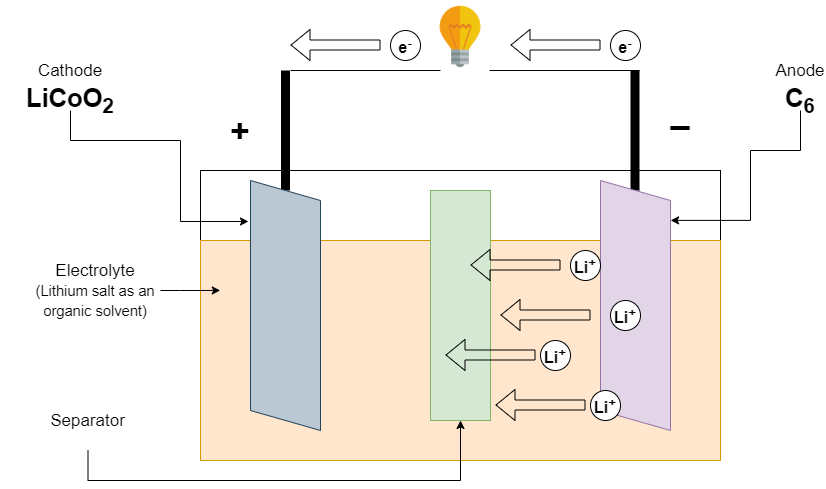
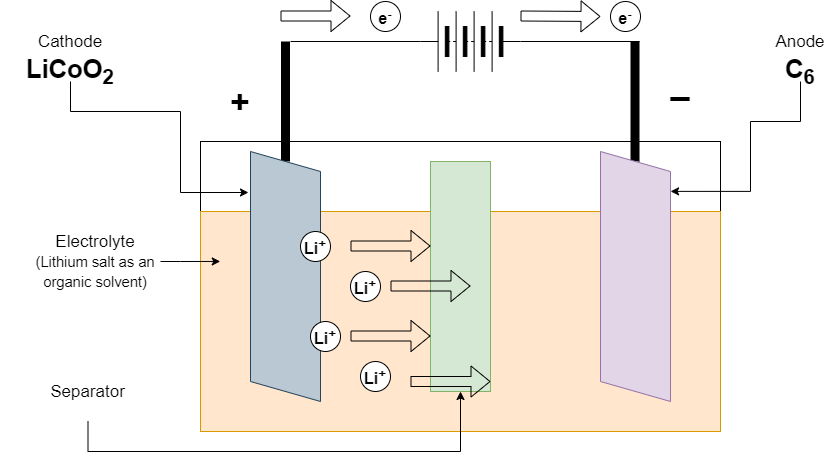
Firstly, a Lithium-ion battery was developed by Akira Yoshino in 1985. The positive electrode (cathode) is made of lithium cobalt oxide and the negative electrode (anode) is made of graphite. Lithium salt as an organic solvent is used as an electrolyte. A separator is used to separate electrodes
This battery has a discharge/charge cycle is about 400 – 1200 cycles. This depends upon various factors, how you are charging or discharging the battery.
The nominal voltage of the lithium-ion battery is 3.60V. When the battery is in full charge the voltage is about 4.2 V. when the battery is fully discharged the voltage is about 3.0V. Li-ion battery comes in different sizes and shapes, the capacity is also available as requirements.
Discharging of Li-ion batteries
At the time of discharge of the battery, the Load is connected to the terminal battery. The lithium-ion is released from the negative electrode and travels to the electrolyte. This lithium-ion is absorbed by a positive electrode. The negative electrode also releases the electrons which travel through an external wire to the positive electrode. This provides us with an electric current to our circuit.
(Discharging of Li-ion battery)
Reaction at the Negative electrode
LiC6 ———-> C6 + Li+ + e–
Reaction at the Positive electrode
CoO2 + Li + e– ———-> LiCoO2
Overall Reaction
LiC6 + CoO2 ———-> C6 + LiCoO2
Li-ion battery can discharge at the rate of 10C (where C is the capacity of the battery ). If your battery can provide 1000mAh then the discharge rate will be 10 x 1000 = 10000mAh.
Charging of Li-ion batteries
At the time of charging the Li-ion battery, The battery is connected to the charger. The Positive electrode losses a negatively charged electron. To maintain this charge balance at the negative electrode, an equal number of positively charged ions are dissolved into the electrolyte solution. These lithium-ion travel over to the positive electrode, where they are absorbed within the graphite. This absorption reaction also deposits electrons into the graphite anode to ‘tie’ up the lithium-ion.
Reaction at the Positive electrode
LiCoO2 ———-> CoO2 + Li + e–
Reaction at the Negative electrode
C6 + Li+ + e– ———-> LiC6
Overall Reaction
C6 + LiCoO2 ———-> LiC6 + CoO2
The Li-ion battery charging chemistries utilize constant current and constant voltage algorithms that can be broken into four parts.
- Trickle Charge:- When the battery is deeply discharged it is below 3.0 V per cell. the constant current of 0.1C maximum used to charge the battery is called trickle charge.
- Constant Current:- When voltage is above 3.0V per cell the constant current is applied in the range of 0.2 C to 1C to perform constant current charging.
- Constant voltage:- when voltage is reached at 4.2 V per cell from constant current charging. The constant voltage is applied till the current taken by the cell drop to zero, this maximizes the performance of the battery.
- Charge Termination:- The end of charging is detected by an algorithm that detects the current range that drops to 0.02C to 0.07C or uses a timer method. It detects when a constant voltage stage starts it terminates the charger after 2 hours of constant voltage stage.
You may also like:
Filed Under: Batteries, Power, Tutorials








Questions related to this article?
👉Ask and discuss on Electro-Tech-Online.com and EDAboard.com forums.
Tell Us What You Think!!
You must be logged in to post a comment.